Metabolism and regulation of metabolite concentration
All cells are surrounded by a lipid bilayer representing a “greasy seal” between the aqueous inside of the cell and the outside. Yet the cells need to import nutrients, water and ions to sustain their internal metabolism to produce energy and the building blocks required to safeguard and replicate the genetic material and the rest of the cellular infrastructure. While only a few molecules are thought to be able to pass through the lipid membrane surrounding cells, most molecules, including vitamins, small molecule hormones, xenobiotics, phytochemicals, pesticides, microbiome metabolites and, importantly, drugs require transporters to enter. The membrane transporters can thus be considered the managers of the interface between chemistry and biology and between organisms and their environment.
As they are overall a large and neglected gene family in humans, we proposed to intensify and coordinate research on the largest group of membrane transporters in the human genome, the SLC solute carrier superfamily (César-Razquin, Snijder et al, Cell 2015). Membrane transporters, and their roles in metabolism, drug transport and signaling are being investigated heavily in the laboratory. A better understanding of the transport and signaling specificity of individual SLCs and their concerted circuits may on one hand lead to better targeted drugs and on the other hand pave the way for an understanding of how biological systems are integrated with their environment.
Nucleoside transporters and purine levels affect epigenetic modulation
Numerous disease development processes are linked to chromatin and epigenetic modulation. One protein involved in chromatin remodeling and identified as an important cancer marker is BRD4. The GSF lab recently showed that the supply of purines, as well as the purine synthesis of a cell can influence BRD4 activity and thus play a role in the carcinogenesis process. In our study (Li et al., Nature Metabolism, 2021), we investigated how purines influence BRD4 and thus the development process of various cancer diseases. With the help of SLCs, we were able to modulate the purine supply and observe the direct effects. We used both a genetic screening approach, based on a transporter-focused CRISPR/Cas9 library, and a drug screening strategy, in collaboration with Stefan Kubicek’s lab, using a compound library mainly consisting of cellular metabolites and drugs, both to track down the modulation of BRD4-dependent chromatin states in myeloid leukemia cells. We showed, on one hand, that inhibiting purine supply as well as disturbing purine synthesis can trigger a functional disturbance of BRD4 and thus impact chromatin accessibility. On the other hand, BRD4 functionality could be restored by adding adenine. These results propose a pharmacologically effective axis between purine metabolism and BRD4-dependent chromatin states. This could be a significant starting point for developing new therapies against BRD4-driven cancer types.
The missing transporter of coenzyme NAD into the mitochondria
Many transporter proteins are still relatively poorly studied and the question of how some nutrients enter and leave cells often remains unanswered. For instance, until recently it was not clear how mitochondria gain access to NAD (nicotinamide adenine dinucleotide), a key metabolite associated with many physiological and pathological processes such as ageing, neurological diseases and the metabolism of cancer cells. The GSF lab, in cooperation with scientists from the University of Bari, have recently identified the protein responsible for the important transport of NAD into mitochondria: the SLC25A51 transporter (Girardi et al., Nature Communications, 2020). In a previous study (Girardi et al., BioRxiv, 2020), we had systematically investigated synthetic interactions between SLCs, starting from a large collection of single knock-out gene in haploid HAP1 cells . Once the specific genes are genetically deleted both individually and in pairs; the effects on cell growth can be measured. By analyzing the genetic interactions of SLC25A51 we could hypothesize a role in mitochondria respiration and redox mechanisms. Metabolomics showed that NAD+ levels were affected. We then showed that SLC25A51 can functionally substitute the known NAD transporter in yeast and vice-versa. The results of our research, which have also been corroborated by two similar independent studies, imply that SLC25A51 was the long sought-after mitochondrial NAD importer and offer the opportunity of modulating the NAD content in this key organelle.
Ligands and chemical probes targeting solute carrier transporters
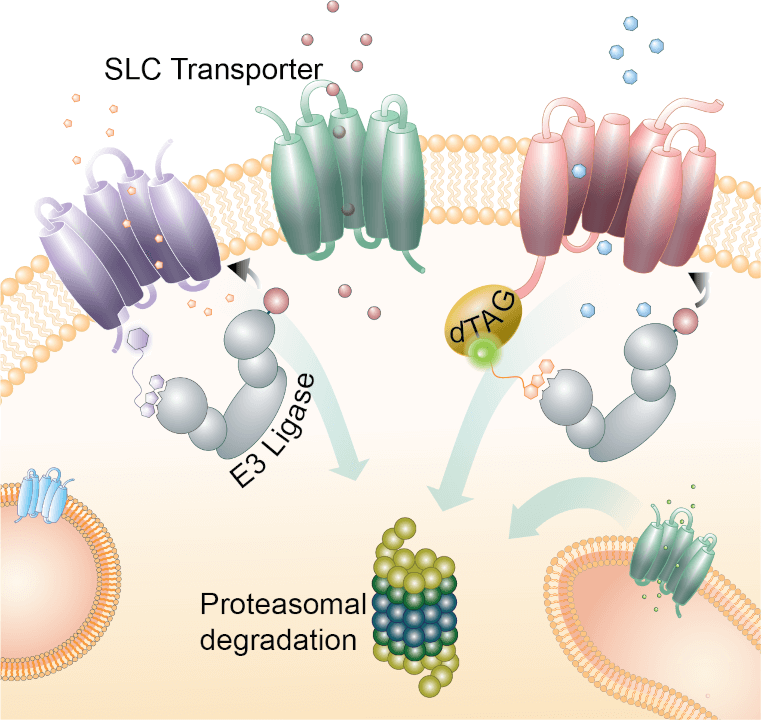
Despite their central roles in cell identity and metabolism, solute carrier (SLC) transporters remain a largely unexploited target class. This gap is reflected in the lack of high-quality chemical ligands or probes and in the small number of compounds that have progressed toward clinical development (reviewed in Casiraghi et al., current opinion in chemical biology, 2021). In our article (Bensimon et al., Cell Chemical Biology, 2020), the GSF lab reports that SLC proteins located in different subcellular compartments are amenable to degradation by ligand-induced proteolysis. Engineering endogenous alleles via the degradation tag (dTAG) technology enabled chemical control of abundance of the transporter proteins. Moreover, we report the new design of a chimeric compound engaging several members of the SLC9 family and leading to their degradation. Our new compound impairs cellular pH homeostasis and promotes cell death in a range of cancer cell lines. These findings open the era of SLC-targeting chimeric degraders and demonstrate potential access of multi-pass transmembrane proteins of different subcellular localizations to the chemically exploitable degradation machinery.
Discovering key components of the nutrients sensing machinery of cells
To decide whether to grow and proliferate or to break down and recycle, cells need to sense if the required nutrients are available. A central role in this context plays the mechanistic target of rapamycin (mTOR) pathway that integrates the presence of growth factors, energy levels, glucose and amino acids to modulate cellular responses, such as protein and lipid synthesis. Past studies have demonstrated that the presence of amino acids is a crucial factor for mTOR activation. Despite this, the precise mechanisms by which amino acid levels are sensed are still poorly understood. The GSF lab, in collaboration with Lukas Huber’s lab from the Medical University Innsbruck and Cesare Indiveri’s lab at the University of Calabria, succeeded in identifying the SLC38A9 transporter as a key factor that mediates mTOR activation. Our study (Rebsamen et al, Nature 2015) contributes to elucidate one of the mechanisms by which the cell recognizes the presence of amino acids and thereby controls mTOR activity. SLC38A9 is the first protein to be shown to physically and directly bind both amino acids and the machinery controlling mTOR function, suggesting it could act as sensor. Our findings may lead to new opportunities to interfere with the mTOR pathway by targeting SLC38A9 in situations where aberrant mTOR activation is thought to promote pathological conditions such as cancer and metabolic disorders.